Next update: 12/15 @ 8 AM CST
Next update: 12/15 @ 8 AM CSTThe role of newer generations of BTK inhibitors in R/R CLL/SLL
Published 12/14/22
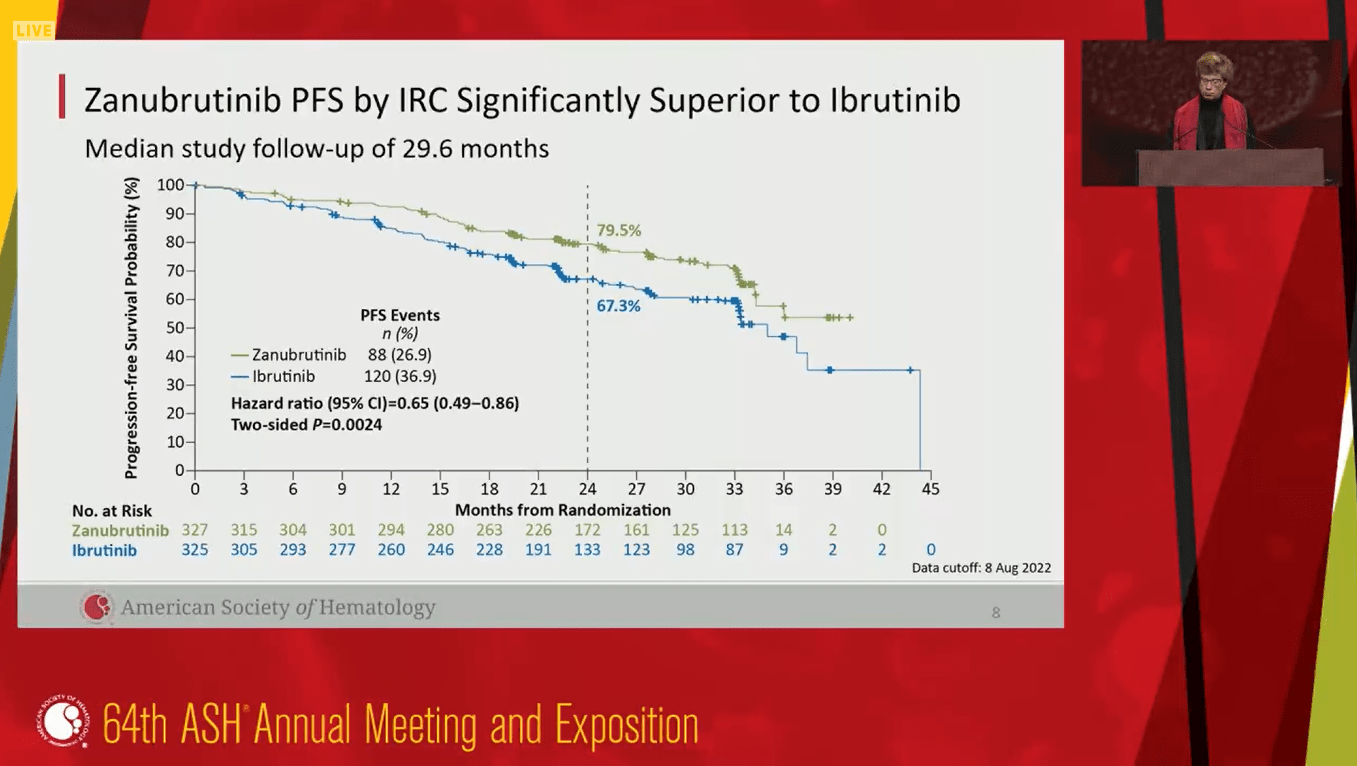
Results from the final analysis of the phase 3 ALPINE trial demonstrated improved PFS and ORR for zanubrutinib vs ibrutinib, the first BTK inhibitor (BTKi) approved for CLL/SLL. The 2-year estimated PFS was 79.5% for zanubrutinib and 67.3% for ibrutinib. Zanubrutinib also showed a favorable safety profile vs ibrutinib, including lower cardiac events. These data suggest that, as the design of BTKis progresses over time, there is potential for improved efficacy and safety over the original BTKi, ibrutinib.
Isatuximab for the treatment of multiple myeloma: Updates from ASH 2022
Published 12/13/22
Sanofi presented multiple studies showcasing new and exciting data for the anti-CD38 antibody isatuximab (SARCLISA) as treatment for multiple myeloma (MM). Here, we discuss three of these presentations: Abstract 247 (ICARIA-MM), Abstract 753 (IKEMA), and Abstract 759 (GMMG-Concept).
As background, isatuximab is currently approved:
- In combination with pomalidomide and dexamethasone, for adult patients with MM who have received ¡Ý2 prior therapies, including lenalidomide and a proteasome inhibitor (based on the ICARIA-MM phase 3 trial)
- In combination with carfilzomib and dexamethasone, for adult patients with relapsed or refractory (RR) MM who have received 1-3 prior lines of therapy (based on the IKEMA phase 3 trial)
These three studies demonstrated encouraging results for isatuximab as a treatment option for (1) relapsed or refractory MM before subsequent treatment (eg, daratumumab), (2) patients with relapsed MM and early or late relapse, and (3) patients with newly-diagnosed, high-risk MM.
ICARIA-MM trial
Dr. Paul Richardson presented updated, longer-term data from a post-hoc analysis evaluating efficacy for isatuximab + pomalidomide + dexamethasone (Isa-Pd) after subsequent therapy in the ICARIA-MM trial. Patients in the Isa-Pd group were still receiving study treatment at data cutoff, so more patients in the Pd group received daratumumab than the Isa-Pd group. Although the immediate use of daratumumab combinations appeared to be less effective after prior anti-CD38 antibody therapy, the depth of response was similar between arms.
Takeaway: There are numerous treatment choices for multiple myeloma, especially in the later-line setting, which makes treatment sequencing challenging. These data provide insight into: (a) treatment sequencing for patients with RRMM who received isatuximab and subsequent daratumumab (eg, potentially using isatuximab earlier in therapy) and (b) the link between treatment sequencing and potential differences in efficacy.
IKEMA trial
Dr. Thomas Martin presented updated results of a post-hoc subgroup analysis of the IKEMA trial. This analysis evaluated efficacy and safety data for isatuximab + carfilzomib + dexamethasone (Isa-Kd) vs carfilzomib + dexamethasone (Kd) in patients with relapsed MM with early vs late relapse. As previously observed in the overall IKEMA trial population, Isa-Kd demonstrated a clinically meaningful improvement in PFS and depth of response for patients with both early and late relapse.
Takeaway: Relapse is common in MM and patients experiencing early relapse have worse survival outcomes. The results of this analysis provide support for the use of isatuximab as a standard of care to treat subgroups of patients with early and late relapses, potentially filing a gap in early relapse.
GMMG-CONCEPT trial
Dr. Katja Weisel presented the exciting results for the preplanned interim analysis of the GMMG-CONCEPT trial, which evaluated MRD negativity rate in patients with high-risk (HR) newly diagnosed (ND) MM treated with isatuximab + carfilzomib + lenalidomide + dexamethasone (Isa-KRd) with or without subsequent autologous stem cell transplant. The trial met its primary endpoint of MRD negativity and reached statistical significance, with an MRD negativity of 67.7% and 54.2% for transplant eligible (TE) and transplant ineligible (TNE) patients, respectively. High and deep response rates were also achieved in both TE and TNE groups.
Takeaway: These data support the use of optimized quadruplet therapy as first-line treatment. This is especially the case for patients with HRMM, who continue to display poorer survival outcomes than patients with no HRMM, even with treatment advances. Finally, there was discussion around the possibility of having this regimen as a choice for the frontline setting, given that this regimen is well-tolerated.
The role of pirtobrutinib in pretreated, relapsed/refractory CLL/SLL
Published 12/13/22
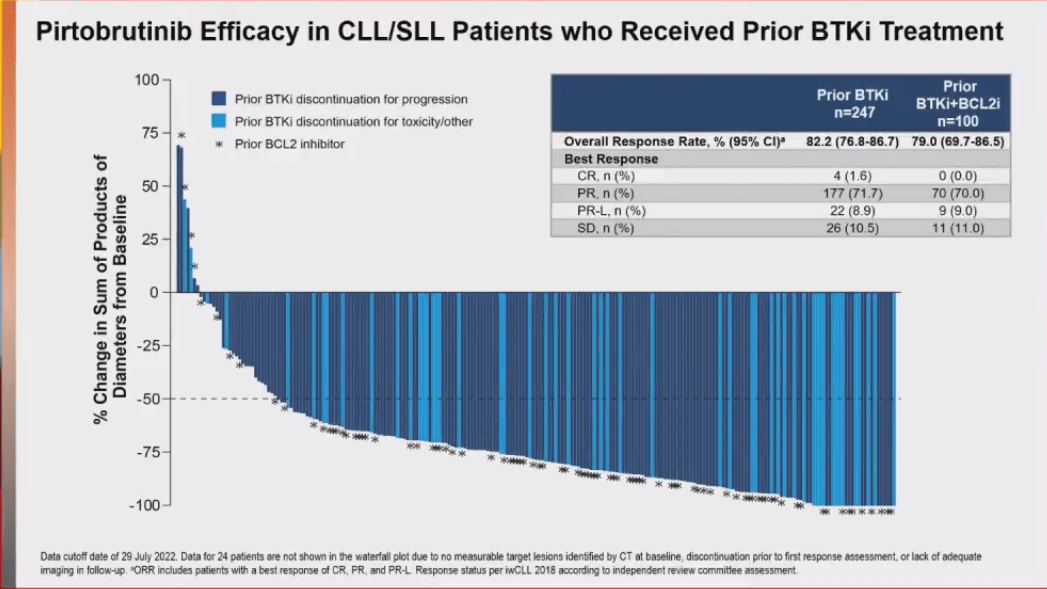
This presentation showed clinically meaningful and durable efficacy of pirtobrutinib in an extended follow-up analysis (2+ years of additional data) of the phase 1/2 BRUIN trial. ORR was 82.2% in patients with CLL/SLL who had received prior BTKi treatment and 79% for those who had received prior BTKi and BCL2i treatment. These data show the role of pirtobrutinib as a potential option in heavily pretreated patients with CLL/SLL who have progressed on BTKi ¡À BCL2i treatment.
Avapritinib As First-Line Therapy in Patients with Advanced Systemic Mastocytosis: Efficacy and Safety from the Pathfinder Clinical Studyv
Published 12/12/22
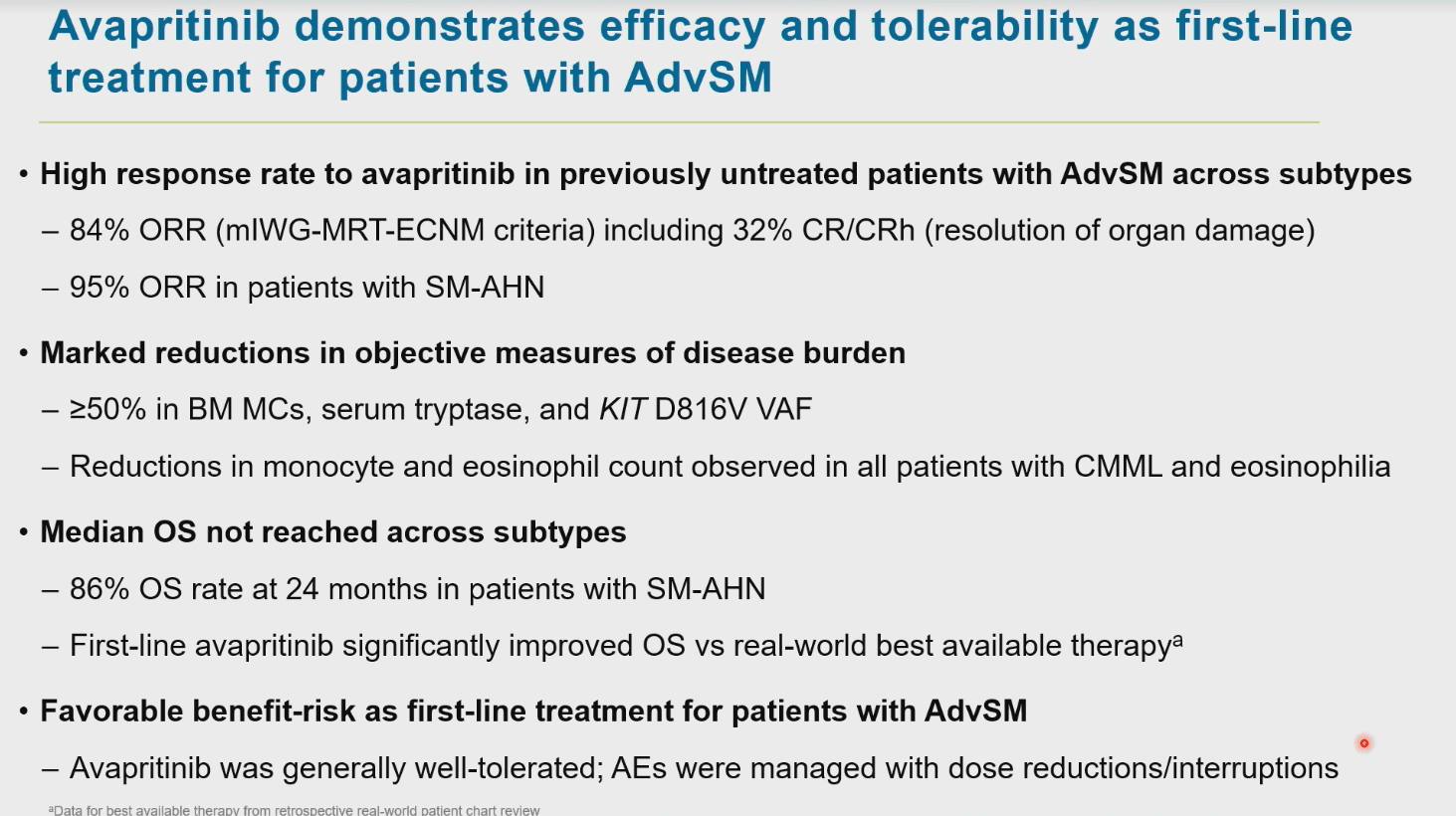
Analysis of avapritinib as 1L therapy for patients with advanced SM demonstrates very high response rates and unprecedented survival, across all subtypes, including those with SM-AHN. The data from the PATHFINDER trial also highlight that avapritinib is well tolerated. This sets a benchmark for 1L treatment of patients with advanced SM
Subcutaneous Epcoritamab + R-Dhax/C in Patients with Relapsed or Refractory DLBCL Eligible for AutoIogous Stem Cell Transplant: Updated Phase 1/2 Results
Published 12/12/22
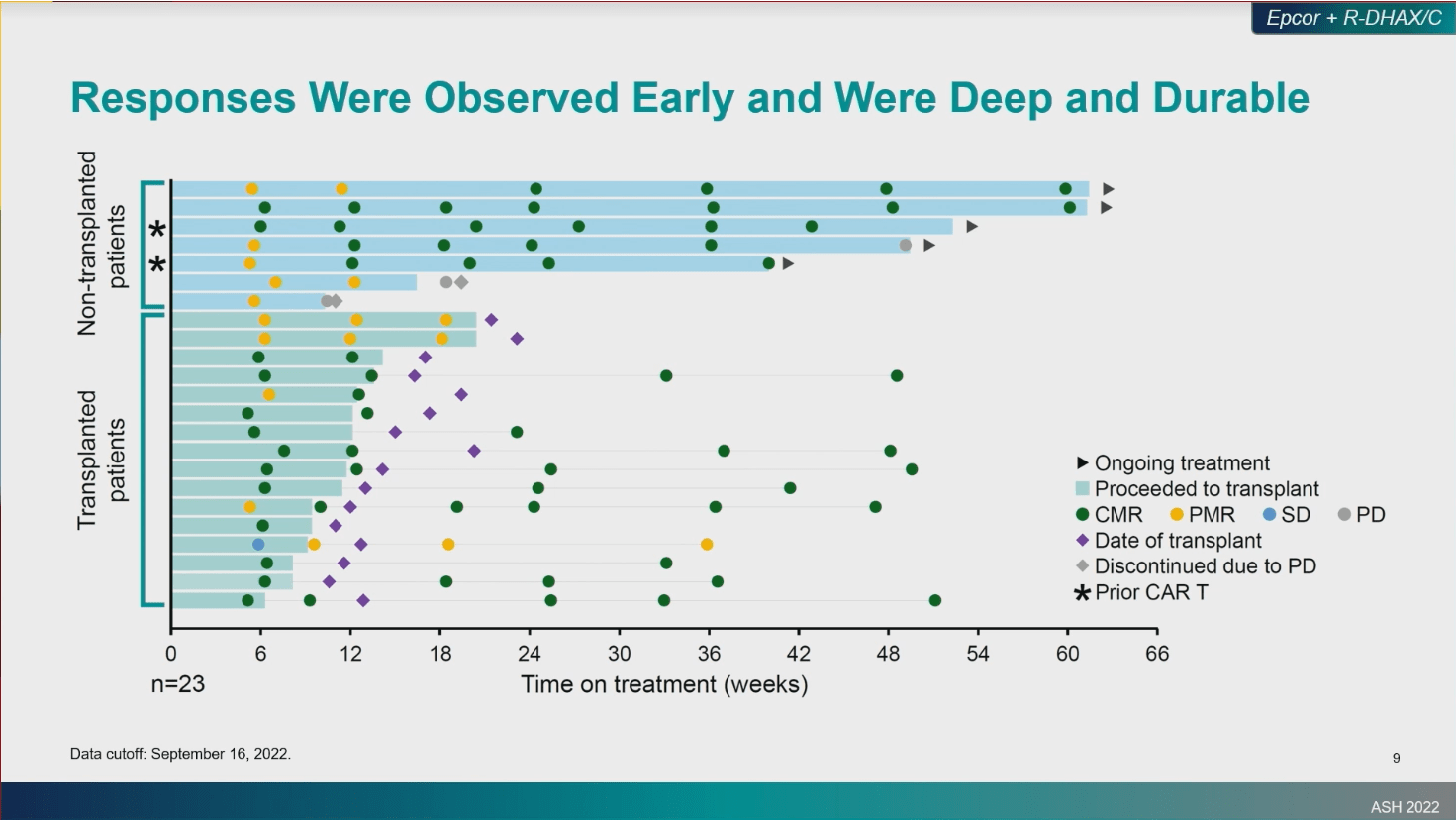
At Sunday’s “Aggressive Lymphomas” session, Abrisqueta et al presented results on sc Epcoritamab + R-DHAC/X in R/R ASCT eligible DLBCL patients. Data from this ECPORE NHL Ph1/2 study in 29 patients showed high responses to therapy that were quick, durable and associated with a manageable safety profile across both transplanted and non-transplanted patients
- ORR=85%; CMR=67%. Median time to response and complete response=1.4 months (mDOR not yet reached)
- Of the 16 patients who proceeded to transplant, none have evidence of disease progression to date
- 4/5 patients remain in CMR at 13.5 month median follow-up
- mOS and mPFS not yet reached at 12.6 month medial follow-up
- All reported CNS were grade 1 or 2
These promising data support Epcoritamab’s future positioning in the DLBCL treatment paradigm as an effective and accessible option for patients who have failed prior therapy. The US FDA has granted Epcoritamab priority BLA review and a PDUFA date is expected in May 2023
BCMA-Targeted T-Cell Engagers Evolving the R/R Multiple Myeloma Treatment Landscape
Published 12/11/22
The multiple myeloma treatment landscape continues to grow with the approval of a number of novel therapies, including several modalities targeting BCMA
- Preliminary Phase 1 data with subcutaneous (SC) alnuctamab demonstrate durable responses (responses deepened over time, with 90% of responses ongoing at data cut-off) and an improved safety profile vs its IV formulation
- While the data are promising, it’s unclear if SC alnuctamab has a clinical advantage over other BCMA-targeted T-cell engagers (eg, TECVAYLI™, elranatamab). Nonetheless, it’s a win to give patients another convenient option
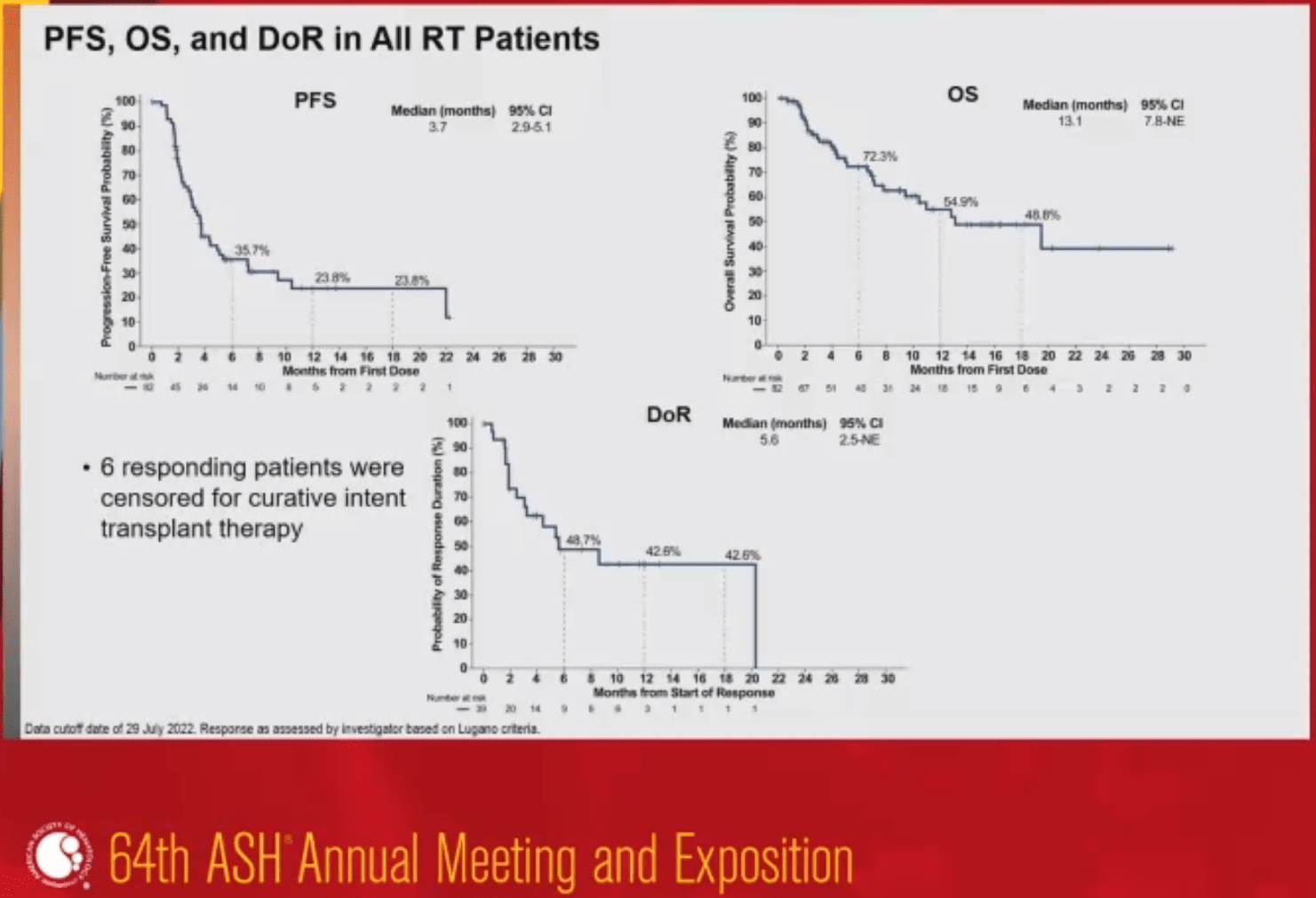
Role of pirtobrutinib in Richter Transformation (RT) in CLL
Published 12/11/22
This is a data set of one of the largest prospective RT populations studied, demonstrating promising efficacy of pirtobrutinib among heavily pretreated patients with RT in CLL
- In all patients with RT, ORR was 52% and median OS was 13 months. This is especially encouraging, given that RT has historically poor prognosis (eg, median OS of 6 months after RT diagnosis).
- These data show that pirtobrutinib may play a significant role in RS, which continues to be an area of high unmet need in CLL
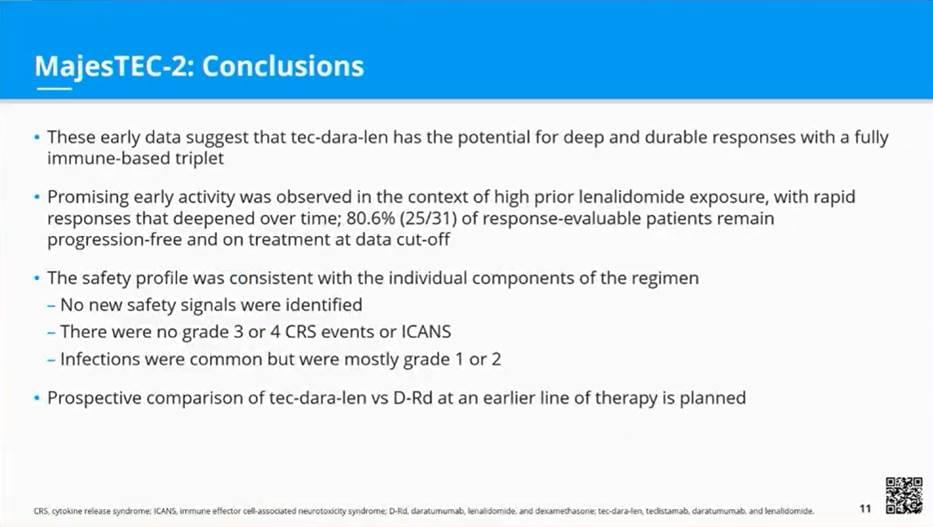
MajesTEC-2: More Options on the Horizon for Treatment of Multiple Myeloma
Published 12/11/22
The multiple myeloma treatment landscape now includes recently approved tec, with more impressive data in combination with widely used dara-len. It will be exciting to see data with tec-dara-len evolve in later stages of trials and in earlier lines of therapy
- Initial phase 1b results demonstrate a high overall response rate (ORR of 93.5% at a median follow-up of 8.4 months) and a well-tolerated safety profile with teclistamab (tec) in combination with daratumumab (dara) and lenalidomide (len)
- Tec appears to enhance immunomodulatory effects of dara-len, while maintaining a consistent safety profile with that of monotherapy and dara-len, potentially giving patients an option with increased disease control but no added safety concerns
Looking forward to the next few days here in New Orleans for ASH 2022! The future of hematology is bright – don’t forget your shades!
Published 12/11/22